Factors that Influence Reactions
It is helpful to identify some general features of a reaction that have a significant influence on its facility. Some of the most important of these are;
A. Energetics: The potential energy of a reacting system changes as the reaction progresses. The overall change may be exothermic (energy is released) orendothermic(energy must be added), and there is usually an activation energy requirement as well. Tables of Standard Bond Energies are widely used by chemists for estimating the energy change in a proposed reaction. As a rule, compounds constructed of strong covalent bonds are more stable than compounds incorporating one or more relatively wea к bonds.
B. Electronic Effects:The distribution of electrons at sites of reaction (functional groups) is a particularly important factor. Electron deficient species or groups, which may or may not be positively charged, are attracted to electron rich species or groups, which may or may not be negatively charged. We refer to these species as electrophiles&nucleophilesrespectively. In general, opposites attract and like repel.
The charge distribution in a molecule is usually discussed with respect to two interacting effects: Aninductive effect, which is a function of the electronegativity differencesthat exist between atoms (and groups); and a resonance effect, in which electrons move in a discontinuous fashion between parts of a molecule.
C. Steric Effects:Atoms occupy space. When they are crowded together, van der Waals repulsions produce an unfavorablesteric hindrance. Steric hindrance may influence conformational equilibria, as well as destabilizingtransition states of reactions.
D. Stereoelectronic Effects: In many reactions atomic or molecular orbitals interact in a manner that has an optimal configurational or geometrical alignment. Departure from this alignment inhibits the reaction.
E. Solvent Effects:Most reactions are conducted in solution, not in a gaseous state. The solvent selected for a given reaction may exzert a strong influence on its course. Remember, solvents are chemicals, and most undergo chemical reaction under the right conditions.
| WORDLIST: | |
| Oxidation | - состояние окисления; валентность, |
| отвечающая за окисление | |
| reduction | - восстановление; раскисление |
| redox | - окислительновосстановительный |
| addition reaction | - реакция соединения; присоединения |
| cyclohexene | - циклогексан |
| peracid epoxidation | - эпоксидирование перкислоты |
| substrate molecule | - основная молекула |
| reaction rate | - скорость реакции |
| kinetics | - динамика; процесс протекания |
| exothermic | - экзотермический |
| endothermic | - эндотермический, теплопоглощающий |
| activation energy | - энергия активации |
| electronic effects | - электронные эффекты |
| electrophile | - элетрофил; молекула, имеющая |
| сходство по электрону | |
| nucleophile | - нуклеофил |
| inductive effect | - индуктивный эффект |
| resonance effect | - явление резонанса |
| steric effects | - стерический(протранственный) |
| эффект | |
| steric hindrance | - пространственная задержка |
EXERCISES:
I.Translate the following information into Russian:
Hydrocarbons are the most reduced compounds of all, with nothing but С and H atoms. Hydrocarbons with single C-C bonds, known as saturated hydrocarbons or alkanes, can be thought of as being built by the snapping together of carbon tetrahedral, with unused carbon bonding positions filled by hydrogen atams.
There are as many different saturated hydrocarbons as there are ways of connecting tetrahedral. The simplest are the straight-chain alkanes (designated "n-"| for "normal").
Methane< ethane, propane, and butane are traditional names for these compounds, but from pentane and hexane onward, the name is derived from the Greek or Latin word for the number of carbons, plus the suffix "-ane" to indicate a saturated hydrocarbons.
II. Translate the following text into English:
Насыщенные углеводороды
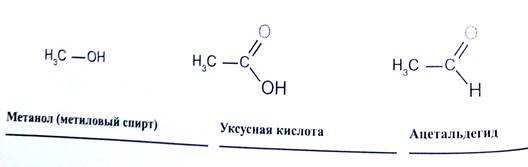
Основные источники насыщенных углеводородов - нефть и природный газ. Реакционная способность насыщенных углеводородов очень низкая, они могут реагировать только с наиболее агрессивными веществами, например, с галогенами или с азотной кислотой. При нагревании насыщенных углеводородов выше 450 С0 без доступа воздуха разрываются связи С-С и образуются соединения с укороченной углеродной цепью. Высокотемпературное воздействие в присутствии кислорода приводит к их полному сгоранию до С02 и воды, что позволяет эффективно использовать их в качестве газообразного (метан — пропан) или жидкого моторного топлива (октан). При замещении одного или нескольких атомов водорода какой-либо функциональной (т.е. способной к последующим превращениям) группой образуются соответствующие производные углеводородов. Соединения, содержащие группировку С-ОН, называют спиртами, НС=0 - альдегидами, СООН- карбоновыми кислотами (слово «карбоновая» добавляют для того, чтобы отличить их от обычных минеральных кислот, например, соляной или серной). Соединение может содержать одновременно различные функциональные группы, например, СООН и NH2, такие соединения называют аминокислотами.
Ненасыщенные углеводороды имеют те же варианты строения основной цепи, что и насыщенные, но содержат двойные или тройные связи между атомами углерода .Простейший ненасыщенный углеводород - этилен.
III. Translate the following material into Russian:
Alkenes also links together or polymerize into long chains - a reaction that is of great importance in plastics and rubbers. When ethylene polymerizes, the double bond opens up to join monomer units into a continuous, saturated hydrocarbon chain, as shown to the right.
Most plastics must have at least 1000 repeating monomer units in each chain before they begin to show familiar "plastic" properties.
Chain lengths usually are not uniform, but the range of lengths can be controlled by the conditions of polymerization. Polyethylene for use in laboratory ware has 5000 to 50,000 carbons per chain. Changing the substituents on the polyethylene chains gives polymers with a variety of properties. Polyvinyl chloride chains, which have as many as 25,000 units, are used for phonograph records and plastic pipe, and with the addition of a plasticizer, as artificial leather.
In this "vulcanization" process, the sulfur atoms cross-link between adjacent poly-isoprene chains and hold them more nearly stationary, opposing any outside stretch or deformation. Soft rubbers contain 1-2% sulfur; hard rubbers may have as much as 35%. Cross-linking of polymer chains is a standard method today of producing a hard, mechanically strong plastic or resin.
LESSON #5
PROPERTIES OF HYDROCARBONS
Hydrocarbons are the most reduced compounds of all, with nothing but С and H atoms. Hydrocarbons with single C-C bonds, known as saturated hydrocarbons or alkanes, can be thought of as being built by the snapping together of carbon tetrahedra, with unused carbon bonding positions filled by hydrogen atoms.
There are as many different saturated hydrocarbons as there are ways of connecting tetrahedra. The simplest are the straight-chain alkanes (designated "n-" for "normal").
Methane, ethane, propane, and butane are traditional names for these compounds, but from pentane and hexane onward, the name is derived from the Greek or Latin word for the number of carbons, plus the suffix "-ane" to indicate a saturated hydrocarbon. The smallest of the hydrocarbons, methane (CH4) through butane (C4H10), are gases, which are familiar as industrial, cooking, and heating fuels.
Methane also is known as marsh gas because some bacteria in swamps can oxidize hydrogen (produced from decomposing matter by other bacteria), using C02 rather than 0„ to yield methane and water - 4H, + C02 —> CH4 + 2^0
The flickering fire of ignited marsh gas in a swamp has been the origin of any number of good tales of ghosts and the supernatural.
The larger the hydrocarbon molecule, the stronger are the van der Waals forces between molecules, and the higher the temperature needed to melt the solid or vaporize the liquid. Pentane (С5H12) through heptadecane (C17H36) are liquids at room temperature, and octadecane (C18H38) and larger molecules are waxy solids.
Polyethylene plastic, which is familiar as a tough, inert but flexible material for laboratory and kitchenware, is a straight-chain hydrocarbon with 5000 to 50,000 carbon atoms per chain.
Polyethylene is tough because the long molecules are entwined around one another and are difficult to unwind and separate.
We use vast quantities of simple hydrocarbons as fuels and lubricants. Natural gas is 85% methane.
Cyclic Hydrocarbons
Saturated hydrocarbons, or alkanes, can form rings as well as straight and branched chains. Open-chain alkanes, straight or branched, have the general formula CJH^,, in which n is the number of carbon atoms.
Id ring alkanes the chain bends back to connect with itself again, and the overall formula is СnH2n
The smallest of these, cyclopropane (С3H6), is strained because the C-C-C angle is 600 which is far less than the optimum of 109.50 for tetrahedral bonding.
The overlap of sp3 orbitals on adjacent carbon atoms is poor, hence the bonds are weak. Cyclobutane (C4H8) and cyclopentane (C5H10) are less strained, and me cyclohex-ane ring (C6H12) has no strain at all.
Cyclohexane can adopt either the "chair" or the "boaf configurations, with the "chair" being favored because it keeps the ends of the molecule farther apart
Unsaturated Hydrocarbons
Hydrocarbons that have double or triple bonds between carbon atoms are called unsaturated hydrocarbons; they are unsaturated in the sense that more hydrogen atoms can be added when H2reacts across the double or triple bonds.
Virtually free rotation exists about a carbon-carbon single bond A methy group (CH3-) can spin like a top about the single bond joining it to another atom.
In contrast, a molecule such as ethylene cannot be twisted about one of its double bonds without breaking the second bond of the double bond. Double bonds are important in defining the geometry of many biologically important molecules, and in helping to make them rigid.
As was mentioned previously, saturated hydrocarbons are called alkanes, and identified by the suffix "ane" in the series methane, ethane, propane, butane, pentane, and hexane, which have one through six carbon atoms, respectively.
Unsaturated hydrocarbons, which have double bonds, are called alkenes and have similar names ending with the suffix "-ene", as in ethene (СД), propene (СД), butene, pentene, and hexene. Ethene, propene, and butene are commonly known as ethylene, propylene, and butylene.
Many oxidations and other useful chemical reactions are spontaneous (accompanied by a decrease in free energy) and exothermic (accompanied by a decrease in enthalpy) and hence are useful as energy sources; yet the reactions often are extremely slow.
We must distinguish between spontaneity and rapidity in chemical reactions. Spontaneous reactions eventually will take place without outside help, but they may take from a microsecond to a billion years to occur.
At room temperature hydrocarbons are spontaneously oxidizable with 0^, but are inert. Heat is required to trigger a reaction.
If an initial heat supply is provided to start the process, then the heat given off by oxidation is enough to keep the reaction going.
Once ignited, combustion is self-sustaining thereafter. A high temperature is needed to overcome the high activation energy (Ea) of the reaction.
Alkanes are relatively unreactive; the term "paraffins" often applied to them means "little affinity."
Saturated hydrocarbons undergo few other reactions. The halogen derivatives are not important for their own sake, but because they are a bridge to other, more useful
compounds.
Once formed, halogen compounds can react to form alcohols, acids, amine bases, and other types of molecules.
Unsaturated hydrocarbons are considerably more reactive than alkanes; their reac-tions take place at moderate temperatures with the help of catalysts.
The Achilles’ heel of alkanes is the double bond, and the main alkene reaction is the addition across this bond of a variety of reagents.
Notice that only one product is formed when an asymmetric reagent such as HCor H2O is used. 2-Chloropropane is formed to the exclusion of 1-chloropropane.
Some plants synthesize the all-trans isomer of polyisoprene, known as guttapercha. Guttapercha is hard and horny rather than rubbery, because the orderly trans-polyisoprene chains can pack next to one another easily in crystalline regions within the polymer.
Simple polymerization of isoprene in the laboratory yields a mixture of cis and trans bonds. More subtle methods of polymerization had to be perfected before a dependable method of making a pure cis polymer was developed in 1955.
As we saw first, 1,3-butadiene is a planar molecule because its double bonds are delocalized along the entire four-carbon chain.
Although the conventional representation shows double bonds between the end pairs of carbon atoms, resonance structures can be drawn in which the central two carbons are double-bonded, and the electron pair of the other double bond is either given to one of the two outside carbon atoms or divided between them.
Twisting about the central carbon-carbon bond is as restricted as twisting about either of the outer carbon-carbon bonds, so all ten atoms of the 1, 3-butadiene molecule are constrained to he in one plane.
Just as three electron pairs are delocalized around six carbon atoms in a benzene ring, so two electron pairs are delocalized along the four-carbon chain of butadiene.
Delocalization can occur whenever single and double bonds alternate along a chain of atoms, so that after all single bonds are formed, each atom along the chain has an unused p orbital and one unused electron. Such molecules are termed conjugated.
WORDLIST:
Saturated hydrocarbon насыщенный (парафиновый) углеводород,
предельный углеводород, алкан
reduced compound простое (упрощенное) вещество
tetrahedral тетраэдрический; четырехгранный
straight chain alkane неразветвленный, прямолинейный алкан
marsh gas болотный газ, метан
heptadecane гептадекан
octadecane октадекан
cyclic hydrocarbon циклический углеводород
ring alkane циклический алкан
| cyclopropane | циклопропан |
| unsaturated hydrocarbon | ненасыщенный(непредельный) |
| углеводород | |
| spontaneous reaction | самопроизвольная, спонтанная |
| реакция | |
| exothermic reaction | экзотермическая реакция |
| enthalpy | теплосодержание, энтальпия |
| activation energy | энергия активации |
| un reactive | нереагирующий,нереактивный; |
| инертный | |
| affinity | химическое сходство |
| plasticizer | пластификатор |
| cross link | поперечная сшивка (связь) |
| resin | смола |
| cis-polymer | цис-полимер |
| planar molecule | плоская молекула |
| benzene ring | бензольное кольцо, |
| ароматическое кольцо | |
| conjugated | сопряженный |
| alkene | алкен |
| covalent bond | ковалентная связь |
EXERCISES:
I. Translate the following sentences into Russian;
a) Methane also is known as marsh gas because some bacteria in swamps can oxidize hydrogen, using C02 rather than 02, to yield methane and water.
b) The larger the hydrocarbon molecule, the stronger are the van der Waals forces between molecules, and the higher the temperature needed to melt the solid or vaporize the liquid.
c) Hydrocarbons that have double or triple bonds between carbon atoms are called unsaturated hydrocarbons.
d) The halogen derivatives are not important for their own sake, but because they are a bridge to other, more useful compounds.
e) If an initial heat supply is provided to start the process, then the heat given off by oxidation is enough to keep the reaction going.
f) Alkenes also link together or polymerize into long chains -a reaction that is of great importance in plastics and rubbers.
g) Polyvinyl chloride chains are used for phonograph records and plastic pipe, and with the addition of a plasticiser, as artificial leather.
h) Cross-linking of polymer chains is a standard method today of producing a hard, mechanically strong plastic or resin.
i) Derealization can occur whenever single and double bonds alternate along a chain of atoms, so that after all single bonds are formed, each atom along the chain has an unused p orbital and one unused electron.
II. Translate the following information into Russian:
These molecules are useful in photosynthesis because they help the plant or bacterium to absorb and harvest light energy.
The relationship between the extent of derealization and wavelength of light absorbed spirilloxanthin, and isorenieratene have successively larger delocalized systems, 11,13, and 15 electron pairs, respectively.
Their electronic energy-level spacings therefore become progressively smaller, as diagrammed within the properties table.
The three molecules absorb in the blue-violet, the green, and the purple-red regions of the visible spectrum, respectively, so the molecules are coloured yellow-orange, purple, and green by the unabsorbed wavelengths.
β-Carotene occurs in all green plants (usually masked by the green of chlorophyll), and is responsible for the yellow colour of carrots, tomatoes, and autumn leaves.
Spirilloxanthin and isorenieratene give purple and green photosynthetic bacteria their characteristic colours.
Simple aliphatic molecules with unconjugated double bonds react quickly with bromine or chlorine to form saturated, dihalogenated molecules:
If the Kekule' structure for benzene were correct, one would expect the same rapid halogenation reaction:
This is not what happens at all. The reaction is slow, and only results in the substitution of first one and then two bromine atoms for hydrogens around the ring, with the delocalized ring structure remaining intact. Hence aromatic molecules have a slowness to react that is more like the behaviour of alkanes than alkenes.
As with the straight-chain hydrocarbons, so with aromatic molecules. The easiest chemical derivatives to prepare are the chlorides and bromides. These are the gateways to the great variety of organic compounds that are the subject of the next chapter.
III. Translate into English:
Способность углерода, соединяться с большинством элементов и образовывать молекулы самого разного состава и строения обусловливает огромное многообразие органических соединений. Эти реакции происходят под воздействием химических связей.
Химическая связь - взаимодействие атомов, обусловливающее их соединение в молекулы и кристаллы. Действующие при образовании химических связей силы имеют в основном электрическую природу. Образование химических связей сопровождается перестройкой электронных уровней связывающихся атомов. Химические связи классифицируются по характеру распределения электронной плотности между атомами, образующими связь, в частности по симметрии распределения и другим признакам. К основным типам химических связей относятся такие как ко-валентные, координационные, ионные, водородные и металлические.
IV. Translate the following text into Russian:
"Town gas" is the name given to the gas with which many Americans cook. Town gas that is produced by the gasification of solid fuels contains - after removal of tar, ammonia and benzene - about 50% hydrogen, 20 to 30% methane and 6 to 17% carbon monoxide( percentages by volume) In addition, the gas contains some carbon dioxide, nitrogen and other impurities. Detoxication consists in removing the carbon monoxide, a highly poisonous colorless and odorless gas. It does not sustain combustion, but burns with a characteristic blue flame.
In order to obtain a nontoxic gas, it is necessary to reduce the carbon monoxide content to between 1 and 1,5%. This can be fairly easy achieved by conversation of carbon monoxide by means of water vapor(steam):
 CO + HIO С0I + HI
CO + HIO С0I + HI
In this process, stream and carbon monoxide, at a temperature of 400o – 480o С and atmospheric pressure, are passed over a catalyst. The town gas flows upwards through a spray tower in which hot water is admitted at the top. As a result, the gas becomes saturated with water vapor.
V. Translate the following information into English:
Химическое строение (порядок соединения атомов в молекулах) простейших алканов - метана, этана и пропана ~ показывают их структурные формулы. Из этих формул видно, что в алканах имеются два типа химических связей:
С-С и С-Н.
Связь С-С является ковалентной неполярной. Связь С-Н - ковалентная слабополярная, т.к. углерод и водород близки по электроотрицательности (2.5 - для углерода и 2.1 - для водорода). Образование ковалентных связей в алканах за счет общих электронных пар атомов углерода и водорода можно показать с помощью
электронных формул:
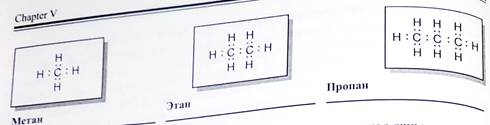
Электронные и структурные формулы отражают химическое строение, но не
дают представления о пространственном строении молекул, которое существенно влияет на свойства вещества.
Пространственное строение, т.е. взаимное расположение атомов молекулы в пространстве, зависит от направленности атомных орбиталей (АО) этих атомов. В углеводородах главную роль играет пространственная ориентация атомных орбиталей углерода, поскольку сферическая ls-AO атома водорода лишена определенной направленности.
Пространственное расположение АО углерода в свою очередь зависит от типа его гибридизации. Насыщенный атом углерода в алканах связан с четырьмя другими атомами. Следовательно, его состояние соответствует 8р3-гибридизации. В этом случае каждая из четырех 5р3-гибридных АО углерода участвует в осевом (а-) перекрывании с s-AO водорода или с sp3-AO другого атома углерода, образуя (7-связи С-Н или С-С.
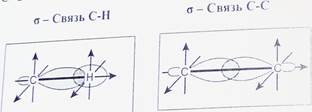
Четыре а-связи углерода направлены в пространстве под углом 109°28', что соответствует наименьшему отталкиванию электронов. Поэтому молекула простейшего представителя алканов - метана СН4 - имеет форму тетраэдра, в центре которого находится атом углерода, а в вершинах - атомы водорода:
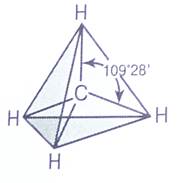
Валентный угол Н-С-Н равен 109o28o. Пространственное строение метана можно показать с помощью объемных (масштабных) и шаростержневых моделей.
CHAPTER VI
REFINERY
Lesson # 1
WHAT IS A REFINERY?
A refinery is a factory. Just as a paper mill turns lumber into legal pads or a glassworks turns silica into stemware, a refinery takes a raw material-crude oil-and transforms it into gasoline and hundreds of other useful products. Inside a maze of silver towers and pipes is a fascinating factory that changes hydrocarbon molecules to make gasoline.
A typical large refinery costs billions of dollars to build and millions more to maintain and upgrade. It runs around the clock 365 days a year, employs between 1,000 and 2,000 people and occupies as much land as several hundred football fields. It's so big and sprawling, in fact, that workers ride bicycles from one station to another.
Chevron has five gasoline-producing "Factories" in the United States and another in Burnaby, British Columbia. Chevron Texaco has refining capacities worldwide of over two million barrels per day.
These world class operations had surprisingly humble origins. In 1876, company pioneers used wagons and mules to haul two primitive stills to a spot near Pico Canyon, Calif., the site of California's first producing oil wells. The stills, each about the size of a garage, were used to heat oil at the prodigious rate of 25 to 40 barrels a day. This "oil boiling" produced kerosene, lubricants, waxes and gasoline--a clear, lightweight liquid that generally was discarded as a useless byproduct Gasoline's lowly status rose quickly after 1892, when Charles Duryea built the first U.S. gas-powered automobile. From then on, the light stuff from crude oil became the right stuff.
Today, some refineries can turn more man half of every 42-gallon barrel of crude oil into gasoline. That's a remarkable technological improvement from 70 years ago, when only 11 gallons of gasoline could be produced. How does this transformation take place? Essentially refining breaks crude oil down into its various components, which then are selectively reconfigured into new products.
This process takes place inside a maze of hardware that one observer has likened to "a metal spaghetti factory." Employees regulate refinery operations from within highly automated control rooms. Because so much activity happens out of sight, refineries are surprisingly quiet places. The only sound most visitors hear is the constant, low hum of heavy equipment
The complexity of this equipment varies from one refinery to the next. In general the more sophisticated a refinery, the better its ability to upgrade crude oil into high-value products. Whether simple or complex, however, all refineries perform three basic steps: separation, conversion and treatment.
Separation: heavy on thebottom, light on the top
Modern separation - which is not terribly different from the "cooking" methods used at the Pico Canyon stills - involves piping oil through hot furnaces. The resulting liquids and vapors are discharged into distillation towers, the tall, narrow columns that give refineries their distinctive skylines.
Inside the towers, the liquids and vapors separate into components or fractions according to weight and boiling point. The lightest fractions, including gasoline and liquid petroleum gas (LPG), vaporize and rise to the top of the tower, where they condense back to liquids. Medium weight liquids, including kerosene and diesel oil distillates, stay in the middle. Heavier liquids, called gas oils, separate lower down, while the heaviest fractions with the highest boiling points settle at the bottom. These tarlike fractions, called residuum, are literally the "bottom of the barrel."
The fractions now are ready for piping to the next station or plant within the refinery. Some components require relatively little additional processing to become asphalt base or jet fuel. However, most molecules that are destined to become high-value products require much more processing.
