Equivalence of Mass and Energy
We know that collisions can result in the production of heat, which is a form of kinetic energy at the molecular level, or the conversion of kinetic energy into entirely different forms of energy, such as light or potential energy.
Let’s consider what happens if a blob of putty moving at velocity v hits another blob that is initially at rest, sticking to it, and as much kinetic energy as possible is converted into heat. (It is not possible for all the KE to be converted to heat, because then conservation of momentum would be violated.) The nonrelativistic result is that to obey conservation of momentum the two blobs must fly off together at v/2.
Relativistically, however, an interesting thing happens. A hot object has more momentum than a cold object! This is because the relativistically correct expression for momentum is p=mgv, and the more rapidly moving molecules in the hot object have higher values of g. There is no such effect in nonrelativistic physics, because the velocities of the moving molecules are all in random directions, so the random motion’s contribution to momentum cancels out.
In our collision, the final combined blob must therefore be moving a little more slowly than the expected v/2, since otherwise the final momentum would have been a little greater than the initial momentum. To an observer who believes in conservation of momentum and knows only about the overall motion of the objects and not about their heat content, the low velocity after the collision would seem to require a magical change in the mass, as if the mass of two combined, hot blobs of putty was more than the sum of their individual masses.
Now we know that mass is invariant, and no molecules were created or destroyed, so the masses of all the molecules must be the same as they always were. The change is due to the change in g with heating, not to a change in m. But how much does the mass appear to change? We have proven that the perceived change in mass exactly equals the change in heat energy between two temperatures, i.e. changing the heat energy by an amount E changes the effective mass of an object by E as well. This looks a bit odd because the natural units of energy and mass are the same. Converting back to ordinary units by our usual shortcut of introducing factors of c, we find that changing the heat energy by an amount E causes the apparent mass to change by m=E/c2. Rearranging, we have the famous E=mc2.
But this whole argument was based on the fact that heat is a form of kinetic energy at the molecular level. Would E=mc2 apply to other forms of energy as well? Suppose a rocket ship contains some electrical potential energy stored in a battery. If we believed that E=mc2 applied to forms of kinetic energy but not to electrical potential energy, then we would have to expect that the pilot of the rocket could slow the ship down by using the battery to run a heater! This would not only be strange, but it would violate the principle of relativity, because the result of the experiment would be different depending on whether the ship was at rest or not. The only logical conclusion is that all forms of energy are equivalent to mass. Running the heater then has no effect on the motion of the ship, because the total energy in the ship was unchanged; one form of energy was simply converted to another.
Example: A rusting nail
Question: A 50-gram iron nail is left in a cup of water until it turns entirely to rust. The energy released is about 0.5 MJ (megajoules). In theory, would a sufficiently precise scale register a change in mass? If so, how much?
Solution: The energy will appear as heat, which will be lost to the environment. So the total mass plus energy of the cup, water, and iron will indeed be lessened by 0.5 MJ. (If it had been perfectly insulated, there would have been no change, since the heat energy would have been trapped in the cup.) Converting to mass units, we have
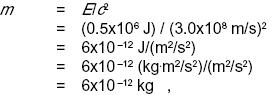
so the change in mass is too small to measure with any practical technique. This is because the square of the speed of light is such a large number in metric units.
In the example we tacitly assumed that the increase in mass would show up on a scale, i.e. that its gravitational attraction with the earth would increase. Strictly speaking, however, we have only proven that energy relates to inertial mass, i.e. to phenomena like momentum and the resistance of an object to a change in its state of motion. Even before Einstein, however, experiments had shown to a high degree of precision that any two objects with the same inertial mass will also exhibit the same gravitational attractions, i.e. have the same gravitational mass. For example, the only reason that all objects fall with the same acceleration is that a more massive object’s inertia is exactly in proportion to the greater gravitational forces in which it participates. We therefore conclude that energy participates in gravitational forces in the same way mass does. The total gravitational attraction between two objects is proportional not just to the product of their masses, m1m2, as in Newton’s law of gravity, but to the quantity (m1+E1)(m2+E2). (Even this modification does not give a complete, self-consistent theory of gravity, which is only accomplished through the general theory of relativity.)
Example: Gravity bending light
The first important experimental confirmation of relativity came when stars next to the sun during a solar eclipse were observed to have shifted a little from their ordinary position. (If there was no eclipse, the glare of the sun would prevent the stars from being observed.) Starlight had been deflected by gravity.
Example: Black holes
A star with sufficiently strong gravity can prevent light from leaving. Quite a few black holes have been detected via their gravitational forces on neighboring stars or clouds of dust.
Since mass and energy are beginning to look like two sides of the same coin, it may not be so surprising that nature displays processes in which particles are actually destroyed or created; energy and mass are then converted back and forth on a wholesale basis. This means that in relativity there are no separate laws of conservation of energy and conservation of mass. There is only a law of conservation of mass plus energy (referred to as mass-energy). In natural units, E+m is conserved, while in ordinary units the conserved quantity is E+mc2.
Example: Electron-positron annihilation
Natural radioactivity in the earth produces positrons, which are like electrons but have the opposite charge. A form of antimatter, positrons annihilate with electrons to produce gamma rays, a form of high-frequency light. Such a process would have been considered impossible before Einstein, because conservation of mass and energy were believed to be separate principles, and the process eliminates 100% of the original mass. In metric units, the amount of energy produced by annihilating 1 kg of matter with 1 kg of antimatter is
E = mc2
= (2 kg)(3.0x108 m/s)2
= 2x1017 J,
which is on the same order of magnitude as a day’s energy consumption for the entire world!
Positron annihilation forms the basis for the medical imaging procedure called a PET (positron emission tomography) scan, in which a positron-emitting chemical is injected into the patient and mapped by the emission of gamma rays from the parts of the body where it accumulates.
Note that the idea of mass as an invariant is separate from the idea that mass is not separately conserved. Invariance is the statement that all observers agree on a particle’s mass regardless of their motion relative to the particle. Mass may be created or destroyed if particles are created or destroyed, and in such a situation mass invariance simply says that all observers will agree on how much mass was created or destroyed.
