Example 2: The reaction between hydrogen peroxide and manganate(VII) ions
Manganate(VII) ions, MnO4-, oxidize hydrogen peroxide, H2O2, to oxygen gas. The reaction is done with potassium manganate(VII) solution and hydrogen peroxide solution acidified with dilute sulphuric acid.
During the reaction, the manganate(VII) ions are reduced to manganese(II) ions.
Let's start with the hydrogen peroxide half-equation. What we know is:

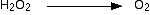
The oxygen is already balanced. What about the hydrogen?
All you are allowed to add to this equation are water, hydrogen ions and electrons. If you add water to supply the extra hydrogen atoms needed on the right-hand side, you will mess up the oxygens again - that's obviously wrong!
Add two hydrogen ions to the right-hand side.

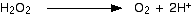
Now all you need to do is balance the charges. You would have to add 2 electrons to the right-hand side to make the overall charge on both sides zero.

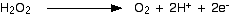
Now for the manganate(VII) half-equation:
You know (or are told) that the manganate(VII) ions turn into manganese(II) ions. Write that down.

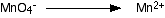
The manganese balances, but you need four oxygens on the right-hand side. These can only come from water - that's the only oxygen-containing thing you are allowed to write into one of these equations in acid conditions.

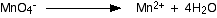
By doing this, we've introduced some hydrogens. To balance these, you will need 8 hydrogen ions on the left-hand side.

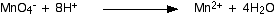
Now that all the atoms are balanced, all you need to do is balance the charges. At the moment there are a net 7+ charges on the left-hand side (1- and 8+), but only 2+ on the right. Add 5 electrons to the left-hand side to reduce the 7+ to 2+.

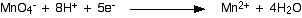
This is the typical sort of half-equation which you will have to be able to work out. The sequence is usually:
· Balance the atoms apart from oxygen and hydrogen.
· Balance the oxygens by adding water molecules.
· Balance the hydrogens by adding hydrogen ions.
· Balance the charges by adding electrons.
Combining the half-reactions to make the ionic equation for the reaction
The two half-equations we've produced are:

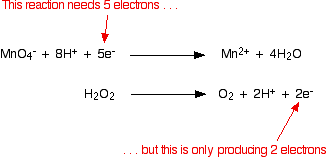
You have to multiply the equations so that the same number of electrons are involved in both. In this case, everything would work out well if you transferred 10 electrons.
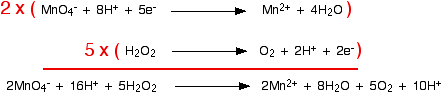
But this time, you haven't quite finished. During the checking of the balancing, you should notice that there are hydrogen ions on both sides of the equation:

You can simplify this down by subtracting 10 hydrogen ions from both sides to leave the final version of the ionic equation - but don't forget to check the balancing of the atoms and charges!

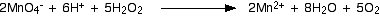
. 
Appendix 2
Laboratory equipment
| 1) 1-63 laboratory apparatus (laboratory equipment)лабораторное оборудование 2) Bunsen burnerгорелка Бунзена 3) gas inlet (gas inlet pipe)подвод газа (газовая подводящая труба) 4) air regulatorрегулятор подвода воздуха 5) Teclu burnerгорелка Теклю 6) pipe unionприсоединение газовой трубы 7) gas regulatorрегулятор поступления газа 8) stemтрубка горелки 9) air regulatorрегулятор поступления воздуха 10) bench torchнастольная горелка 11) oxygen inletподвод кислорода 12) hydrogen inletподвод водорода 13) oxygen jetструя кислорода 14) tripodтреножник, тренога 15) ring (retort ring)кольцо для реторты 16) funnelворонка 17) pipe clay triangleтрубчатый глиняный треугольник 18) wire gauzeпроволочная сетка 19) wire gauze with asbestos centre (Am. center)проволочная сетка с асбестовым центром 20) beakerстакан 21) burette (for delivering measured quanti- ties of liquid)бюретка (для выпуска измеренных объемов жидкости) 22) burette standштатив для бюреток 23) burette clampзажим для бюреток 24) graduated pipetteградуированная пипетка 25) pipetteпипетка 26) measuring cylinder (measuring glass)мерный цилиндр (измерительный стакан) 27) measuring flaskмерная колба 28) volumetric flaskмерная колба 29) evaporating dish (evaporating basin), made of porcelainвыпарная чашка, выполненная из фарфора 30) tube clamp (tube clip, pinchcock)зажим для трубок 31) clay crucible with lidглиняный тигель с крышкой 32) crucible tongsтигельные щипцы 33) clampструбцина | 34) test tubeпробирка 35) test tube rackштатив для пробирок 36) flat-bottomed flaskплоскодонная колба 37) ground glass neckгорлышко с притертой стеклянной пробкой 38) long-necked round-bottomed flaskдлинногорлая круглодонная колба 39) Erlenmeyer flask (conical flask)колба Эрленмайера (коническая колба) 40) filter flaskколба для фильтрования под вакуумом 41) fluted filterгофрированный фильтр 42) one-way tapодноходовый кран 43) calcium chloride tubeтрубка с хлоридом кальция 44) stopper with tapпробка с краном 45) cylinderцилиндр 46) distillation apparatus (distilling apparatus)перегонный аппарат 47) distillation flask (distilling flask)перегонная колба 48) condenserконденсатор 49) return tap, a two-way tapвозвратный кран, двухходовой кран 50) distillation flask (distilling flask, Claisen flask)перегонная колба (вакуум-перегонная колба, колба Кляйзена) 51) desiccatorэксикатор (сушилка) 52) lid with fitted tubeкрышка с вставленной трубкой 53) tapкран 54) desiccator insert made of porcelainфарфоровый вкладыш в эксикаторе 55) three-necked flaskтрехгорлая колба 56) connecting piece (Y-tube)соединительная (Y-образная) трубка 57) three-necked bottleтрехгорлая склянка 58) gas-washing bottleсклянка 59) gas generator (Kipp's apparatus, Am. Kipp generator)генератор газа 9аппарат Кипа, генератор Кипа) 60) overflow containerпереточный сосуд 61) container for the solidсосуд для засыпки реагента 62) acid containerсосуд для кислоты 63) gas outletтрубка для выпуска газа |
Appendix 3
