Improving the quality of petroleum products
Catalysts also help individual hydrocarbon molecules to change their structures, processes called isomerization and reforming. Long unbranched paraffin molecules and cycloparaffins aren't useful in gasoline because they cause knocking. Isomeriz-ing catalysts, usually platinum, help the unbranched molecules to rearrange into highly branched molecules (Fig. 1). Reforming catalysts, usually platinum and rhenium, assist in converting cycloparaffins into aromatics. In both cases, the octane numbers increase substantially. Much of the low octane, raw gasoline obtained from the first distillation tower is subsequently sent through catalytic isomerizing and reforming facilities to increase its octane number.
The goal of isomerization is to add more branches to a paraffin molecule by interchanging hydrogen atoms and carbon atoms. On the isomerizing catalyst's surface, one carbon atom and one hydrogen temporarily let go of the hydrocarbon molecule and exchange places. Several such interchanges turn the molecule into highly branched paraffin with a high octane number.
Without a catalyst, this isomerizing process requires a great deal of energy. Two separate covalent bonds must break completely so that the pieces become free radicals. The carbon and hydrogen atoms must then exchange places and reattach to the main portion of the molecule. This complicated process is unlikely to happen, even at high temperatures.
The isomerizing catalyst facilitates the process by binding temporarily to the molecule and its fragments. The various pieces never become free radicals. Instead, they migrate along the surface of the catalyst and eventually reattach to one another without ever being completely free. The catalyst even helps the fragments stay close enough together to exchange places. What would otherwise be an almost impossible event becomes rather likely.
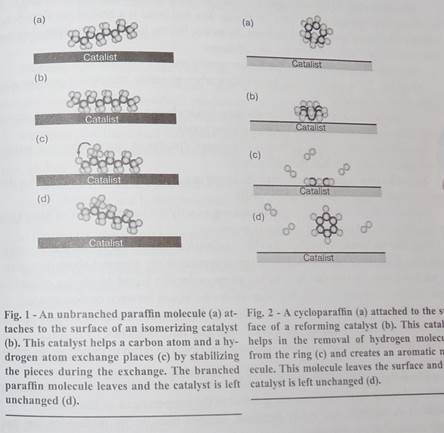
A reforming catalyst helps cycloparaffin molecules get rid of hydrogen atoms and become aromatics (Fig, 2), Aromatics have higher octane numbers than cycloparaffins, so this reforming is important for gasoline. Although catalysts ease the removal of the hydrogen atoms as hydrogen molecules, the final product molecules have more chemical potential energy than the original molecules. Because this reaction converts a significant amount of thermal energy into chemical potential energy, heat must be added to keep it going.
In addition to isomerization and reforming, oil refineries also use Cfttalyststo attach smaller molecules together to form larger molecules. Catalytic akylation and polymerization are used to form gasoline molecules from smaller molecules that would otherwise be difficult to use. Both processes start with olefin molecules produced in thermal or catalytic cracking. The olefin molecules have reactive double bonds, and catalysts encourage them to stick to one another or to other molecules. These reactions produce highly branched, high octane gasoline.
WORDLIST
Isomerization изомеризация
branched paraffin предельный парафин
rhenium рений
covalent bond ковалентная связь
to reattach восстановить (соединение)
original molecule исходная молекула
reactive double bond химически активная двойная связь
catalytic alkylation каталитическое алкирование
absorption column абсорбционная колонна
scrubber газапромыватель
GLOSSARY
aromatic ring A ring of six carbon atoms in which each carbon atom is bound to its neighbor with one and a half covalent bonds. The first bond is a typical covalent bond, with a pair of electrons located between the atoms, but the half bond involves electrons that circulate above and below the ring of atoms.
aromatics Hydrocarbon molecules containing one or more aromatic rings.
crackingThe separation of a large hydrocarbon molecule into two or more smaller
hydrocarbon molecules.
cycloparaffins Ring-like hydrocarbon molecules based on closed chains of carbon
atoms. These rings contain only single covalent bonds.
immiscible Two or more materials that cannot dissolve in one another and remainas two separate phases when they are mixed.
isomerization A rearrangement of the atoms in a molecule that leaves it with a different spatial structure than it had originally.
IsomersTwo or more molecules containing exactly the same number of atoms but having different and inequivalent spatial structures.
nonpolar molecule A molecule that has no electrical dipole. A nonpolar molecule has no positive or negative end because its charge is symmetrically arranged about its center.
olefins Chain-like or ring-like hydrocarbon molecules based on chains of carbon atoms. Olefin molecules include one or more double bonds between carbon atoms.
paraffins Chain-like hydrocarbon molecules based open chains of carbon atoms. Paraffins contain only single covalent bonds and may have branches.
reforming Removal of hydrogen atoms from a cycloparaffin to create an aromatic hydrocarbon.
residual liquid The liquid that falls to the bottom of a distillation tower because it contains molecules that rarely become gaseous at the temperatures of the tower.
volatile A material that easily becomes gaseous because its molecules are not strongly attached to one another.
EXERCISES
I. Give equivalents to the following words and word combinations:
Hydrogen atom, branched paraffin, ароматические вещества, raw gasoline, непредельные углеводороды, isomerizaton, facilities, октановое число, catalytic cracking, высококачественный бензин, двойные связи, reforming catalyst.
каталитическое алкилирование абсорбционная колонна газопром ы вате л ь
//. Answer the following questions:
a) How is it possible to raise the quality of a raw gasoline?
b) What is the isomerization?
c) Is there any difference between the isomerization and reforming?
d) What is the task of a catalyst in the isomerizing process? g) What is the raw material for the catalytic alkylation?
III. Translate into Russian:
Treating and Blending the FractionsDistillated and chemically processed fractions are treated to remove impurities, such as organic compounds containing sulfur, nitrogen, oxygen, water, dissolved metals and inorganic salts. Treating is usually done by passing the fractions through the following:
a column of sulfuric acid - removes unsaturated hydrocarbons (those with carbon-carbon double-bonds), nitrogen compounds, oxygen compounds and residual solids (tars, asphalt)
an absorption column filled with drying agents to remove water
sulfur treatment and hydrogen-sulfide scrubbers to remove sulfur and sulfur compounds
After the fractions have been treated, they are cooled and then blended together to
make various products, such as:
gasoline of various grades, with or without additives
lubricating oils of various weights and grades (e.g. 10W-40,5W-30)
kerosene
jet fuel
diesel fuel
heating
oil
chemical of various grades for making plastics and polymers
IV. Translate into English:
По составу автомобильные бензины представляют собой смесь компонентов, получаемых в результате различных технологических процессов: прямой перегонки нефти, каталитического риформинга, каталитического крекинга и гидрокрекинга вакуумного газойля, изомеризации прямогонных фракций, алкилирова-ния, ароматизации, термического крекинга и коксования. Компонентный состав бензина зависит, в основном, от его марки и определяется набором технологических установок на нефтеперерабатывающем заводе.
Базовым компонентом для выработки автомобильных бензинов являются обычно бензины каталитического реформинга или каталитического крекинга.
Бензины каталитического реформинга характеризуются низким содержанием серы, в их составе практически отсутствуют олефины, поэтому они высокостабильны при хранении. Однако повышенное содержание в них ароматических углеводородов с экологической точки зрения является лимитирующим фактором. К их недостаткам также относится неравномерность распределения детонационной стойкости по фракциям. В составе бензинового фонда России доля компонента каталитического реформинга превышает 50 %.
Бензины каталитического крекинга характеризуются низкой массовой долей серы, октановыми числами по исследовательскому методу 90-93 единицы. Содержание в них ароматических углеводородов составляет 30-40 %, олефиновых — 25-35 %. В их составе практически отсутствуют диеновые углеводороды, поэтому они обладают относительно высокой химической стабильностью (индукционный период 800-900 мин.). По сравнению с бензинами каталитического реформинга для бензинов каталитического крекинга характерно более равномерное распределение детонационной стойкости по фракциям.
V. Translate into Russian:
